The printed electrode for blood sugar reagent strips is one of the core components of blood sugar detection. Its principle involves the combination of electrochemical detection technology and biological enzyme-catalyzed reactions. By printing the electrode on the substrate material according to the design drawings, rapid and accurate measurement of the glucose concentration in blood is achieved. measurement. The following explains the structure, working principle and key technical details:
1. Basic structure of printed electrode
The printed electrode of a blood sugar reagent strip is usually composed of a substrate material and a multi-layer printed electrode pattern. The core structure includes:
1. base material
Most of them are polymer materials with good insulation (such as polyvinyl chloride PVC, polyester PET, etc.), which play a role in supporting electrodes and insulation.
2. electrode system
Conductive materials (such as carbon paste, silver/silver chloride paste) are printed on the substrate through screen printing, inkjet printing and other technologies to form three key electrodes:
1)Working Electrode (WE): The core area where glucose oxidation occurs. Biological enzymes such as glucose oxidase (GOD) or glucose dehydrogenase (GDH) are usually immobilized on the surface.
2)Reference Electrode (RE): Provides a stable reference potential to ensure the accuracy of the working electrode potential (commonly used silver/silver chloride Ag/AgCl).
3)Counter Electrode (CE): It forms a current loop with the working electrode to balance electron transfer and avoid polarization of the reference electrode.
3. reagent layer
Covering the surface of the working electrode, it contains biological enzymes (catalyzes the glucose reaction), electronic mediators (such as potassium ferricyanide, ferrocene, etc., which transfer electrons) and buffer solution (maintains the pH of the reaction environment stable).
2. Core working principle: electrochemical oxidation-detection cycle
When a blood sample is dropped onto the reagent strip, complete the blood sugar concentration test by using the following steps:
1. Enzyme-catalyzed oxidation of glucose
Glucose in the blood diffuses into the reagent layer and undergoes oxidation reactions under the catalysis of biological enzymes:
Take glucose oxidase (GOD) as an example:
Glucose + O ˇ → Gluconic acid + H ˇ O ˇ (catalyzed by GOD)
If glucose dehydrogenase (GDH) is used, the reaction is independent of oxygen and directly oxidizes glucose to gluconate lactone and simultaneously oxidizes coenzymes (such as NAD → NADH).
2. Electron transfer and current generation
The electrons produced by the oxidation reaction need to be transferred to the working electrode through an electron mediator (to avoid errors caused by direct reaction with oxygen):
·For example, H2O2 is oxidized to O ˇ on the surface of the working electrode, releasing electrons (H2O2 → 2H + O ˇ +2e);
·An electron mediator (such as potassium ferricyanide [Fe (CN)] ³) accepts electrons and is reduced to [Fe (CN)] 4, and is then re-oxidized to [Fe (CN)] ³ on the surface of the working electrode. At the same time, electrons are released to the electrode to form an electric current.
3. Correlation between current signals and blood sugar concentration
A constant voltage (controlled by the instrument, usually 0.2 - 0.5V) is applied between the working electrode and the counter electrode, and the intensity of the oxidation current generated by electron transfer is proportional to the glucose concentration (obeys Faraday's law):
After the instrument detects the current signal, it converts the current value into a glucose concentration (mmol/L or mg/dL) through an internal calibration curve and displays the result.
3. The key role of printing technology
The core advantages of printing technology (such as screen printing) are:
1. Low-cost, large-scale production: The electrode pattern is quickly copied to the substrate through a template, suitable for mass manufacturing.
2. Miniaturization and integration: The electrode size can be accurately controlled at the micron level, reducing the amount of sample (only 1 - 5 μ L of blood is needed).
3. Improved stability: The printed electrode material (such as carbon paste) is closely combined with the reagent layer to ensure stable electron transfer efficiency.
4. Technical characteristics and advantages
·Rapid detection: The entire reaction and detection process can be completed in 10 seconds to 30 seconds.
·High specificity: Biological enzymes only catalyze glucose, reducing interference from other substances.
·Portability: With portable blood glucose meters, suitable for home or point-of-the-point testing (POCT) scenarios.
Summary
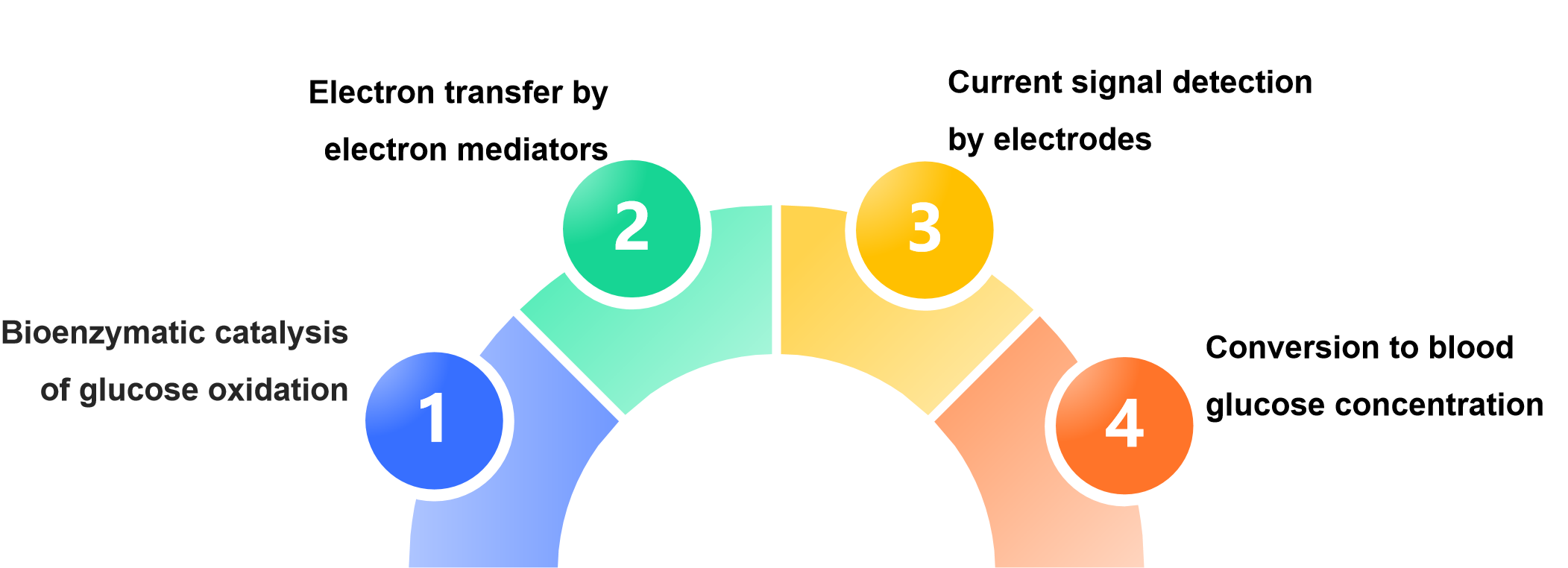
The principle of printed electrode of blood glucose reagent strip is:
The printing process provides an efficient and low-cost miniaturized electrode carrier for this process, which is the key basis for the popularization of modern blood glucose detection technology.
The principle of printed electrodes in blood glucose test strips
I. Basic Structure of Printed Electrodes
A blood glucose test strip's printed electrode typically consists of a substrate material and multi-layer printed electrode patterns, with the core structure including:
1. Substrate material
Mostly high molecular materials with good insulation (such as polyvinyl chloride PVC, polyester PET, etc.), which serve to support the electrodes and provide insulation.
2. Electrode system
Conductive materials (such as carbon paste, silver/silver chloride paste) are printed on the substrate through screen printing, inkjet printing and other technologies to form three key electrodes:
1. Working Electrode (WE):The core area where glucose oxidation reactions occur, with bioenzymes such as glucose oxidase (GOD) or glucose dehydrogenase (GDH) usually immobilized on its surface.
2. Reference Electrode (RE):Provides a stable reference potential to ensure the accuracy of the working electrode's potential (silver/silver chloride Ag/AgCl is commonly used).
3. Counter Electrode (CE):Forms a current loop with the working electrode to balance electron transfer and prevent polarization of the reference electrode.
3. Reagent layer
Covering the surface of the working electrode, it contains bioenzymes (catalyzing glucose reactions), electron mediators (such as potassium ferricyanide, ferrocene, etc., for electron transfer), and buffer solutions (maintaining stable pH in the reaction environment).
II. Core Working Principle: Electrochemical Oxidation-Detection Cycle
When a blood sample is dropped onto the test strip, the blood glucose concentration is measured through the following steps:
1. Enzymatic catalytic oxidation of glucose
Glucose in the blood diffuses into the reagent layer and undergoes oxidation reaction under the catalysis of bioenzymes:
· Taking glucose oxidase (GOD) as an example:
Glucose + O₂ → Gluconic acid + H₂O₂ (catalyzed by GOD)
· If glucose dehydrogenase (GDH) is used, the reaction does not depend on oxygen, directly oxidizing glucose to gluconolactone while oxidizing coenzymes (such as NAD⁺→NADH).
2. Electron transfer and current generation
The electrons generated by the oxidation reaction need to be transferred to the working electrode through electron mediators (to avoid errors caused by direct reaction with oxygen):
· For example, H₂O₂ is oxidized to O₂ on the surface of the working electrode, releasing electrons (H₂O₂ → 2H⁺ + O₂ + 2e⁻);
· Electron mediators (such as potassium ferricyanide [Fe(CN)₆]³⁻) accept electrons and are reduced to [Fe(CN)₆]⁴⁻, which is then re-oxidized to [Fe(CN)₆]³⁻ on the surface of the working electrode, releasing electrons to the electrode to form a current.
3. Relationship between current signal and blood glucose concentration
A constant voltage (controlled by the instrument, usually 0.2~0.5V) is applied between the working electrode and the counter electrode. The oxidation current intensity generated by electron transfer is proportional to the glucose concentration (following Faraday's law):
· After detecting the current signal, the instrument converts the current value into glucose concentration (mmol/L or mg/dL) through an internal calibration curve and displays the result.
III. Key Role of Printing Technology
The core advantages of printing technologies (such as screen printing) are:
1. Low cost and mass production: The electrode pattern can be quickly copied onto the substrate through templates, suitable for mass manufacturing.
2. Miniaturization and integration: The electrode size can be precisely controlled at the micrometer level, reducing sample usage (only 1~5μL of blood is needed).
3. Improved stability: The printed electrode materials (such as carbon paste) are tightly combined with the reagent layer, ensuring stable electron transfer efficiency.
IV. Technical Features and Advantages
· Rapid detection: The entire reaction and detection process can be completed within 10~30 seconds.
· High specificity: Bioenzymes only catalyze reactions with glucose, reducing interference from other substances.
· Portability: Compatible with portable blood glucose meters, suitable for home or point-of-care testing (POCT) scenarios.
Summary
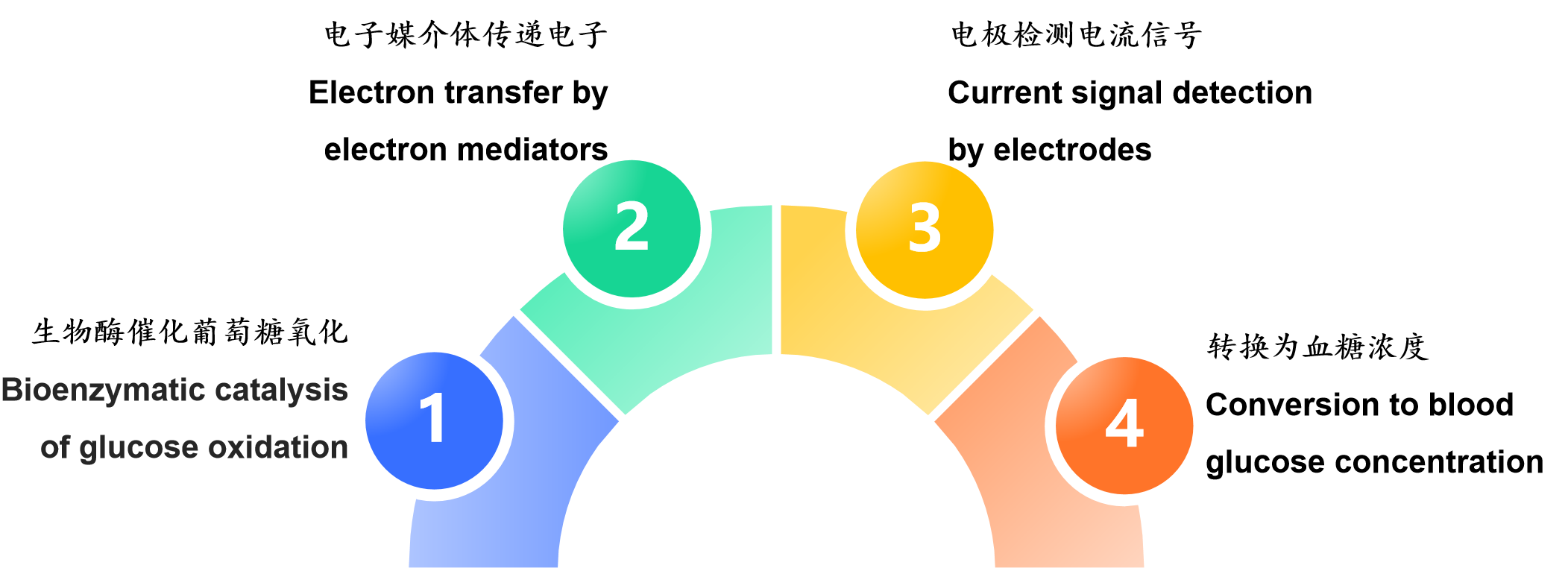
The principle of printed electrodes in blood glucose test strips is an electrochemical process of "bioenzymatic catalysis of glucose oxidation → electron transfer by electron mediators → electrode detection of current signals → conversion to blood glucose concentration". The printing process provides an efficient and low-cost miniaturized electrode carrier for this process, which is the key foundation for the popularization of modern blood glucose detection technology.